Cancer epidemiology
In Poland, malignant tumors constitute a growing health, social and economic problem. The number of new cases is approximately 170,000 and the number of deaths exceeds 100,000. Currently, over 1.17 million Poles live with cancer. In the second decade of the 21st century, it is estimated that every year for every 100,000 people in the Polish population, approximately 440 people were diagnosed with cancer, and over 3,000 people were living with cancer diagnosed in the last 10 years. The incidence of cancer increases exponentially with age, increasing 10-fold every two/three decades of life. Cancer is the second most common cause of death in Poland, accounting for approximately a quarter of all deaths. The number of cancer cases and deaths has increased approximately 2.5 times since the mid-1960s. The most common cancer among men is prostate cancer and among women, breast cancer. Colorectal cancer also occurs frequently in both sexes.
Oncological disease, which is one of the most serious health problems in the world, generates enormous tensions, both for the individual and for society. For the patient, a diagnosis of "cancer" brings fear and uncertainty, and for the doctor - the challenge of designing the most effective treatment plan. Over the past decades, scientists around the world have conducted a number of studies aimed at better understanding cancer, developing more effective diagnostic methods and improving treatments. During this time, rapid progress in the field of oncology has been noticeable, but despite this, new solutions are still needed. One of the most promising is liquid biopsy.
What is a liquid biopsy?
Liquid biopsy in oncology is the equivalent of a classic biopsy, with the difference that the genetic material for analysis is taken from the patient's blood and not directly from the tumor.
Liquid biopsy is an innovative method for detecting cancer biomarkers that can be found directly in biological fluids (e.g. peripheral blood).
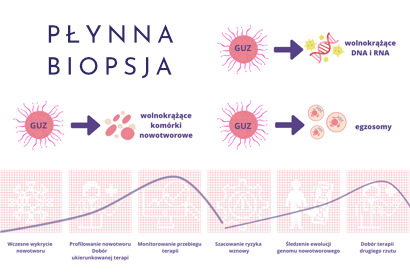
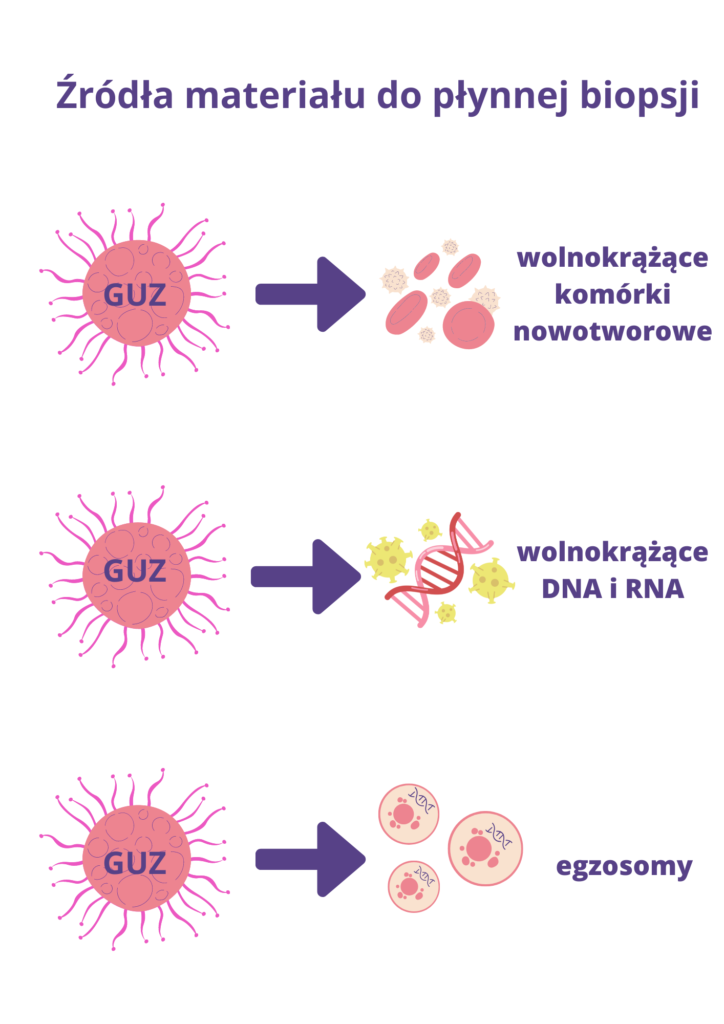
Material for research may come from several sources:
- Freely circulating tumor cells (CTCs). These are cancer cells that have broken away from the tumor and entered the patient's bloodstream, detected in various types of cancer, generally in the later stages of the development of the cancer process, in malignant tumors. They are less used in early diagnosis, but more used as a predictive marker and in the selection of targeted drugs.
- Free circulating DNA (cfDNA – circulating free DNA) analysis of this type of material is possible because all cells, including cancer cells, release genetic material into the bloodstream when they die. Genetic material is released from cancer cells, which undergo the processes of apoptosis and necrosis (cell death). These are small fragments of DNA, called free, free-circulating or free-circulating DNA (ctDNA(cell-free circulating tumor DNA). Moreover, the concentration of free-circulating tumor DNA in the patient's blood increases as the tumor grows. This suggests that a simple measurement of the level of ctDNA in a patient's blood can be used to determine the stage of cancer and monitor the effectiveness of therapy.
The analysis of free-circulating DNA isolated from blood plasma may also enable the detection and characterization of cancer at a very early stage, before it could be detected by other diagnostic methods. Moreover, it can serve as a predictive marker and a source of the so-called liquid biopsy to assess the advancement of neoplastic processes and monitor the course of treatment.
- Free circulating RNA (cfRNA)
Released into the bloodstream, similarly to DNA, in the process of necrosis and apoptosis of cancer cells. For several years, the fraction of free-circulating micro-RNA (cfmiRNA) - short molecules (approx. 22-nucleotides long) that may be specific for various types of cancer - has attracted particular interest. - Exosomes
Exosomes are small, round vesicles containing min. proteins, RNA, DNA, miRNA and metabolites released, among others by cancer cells and detected in the patient's peripheral blood.
Advantages of liquid biopsy over traditional (surgical) biopsy
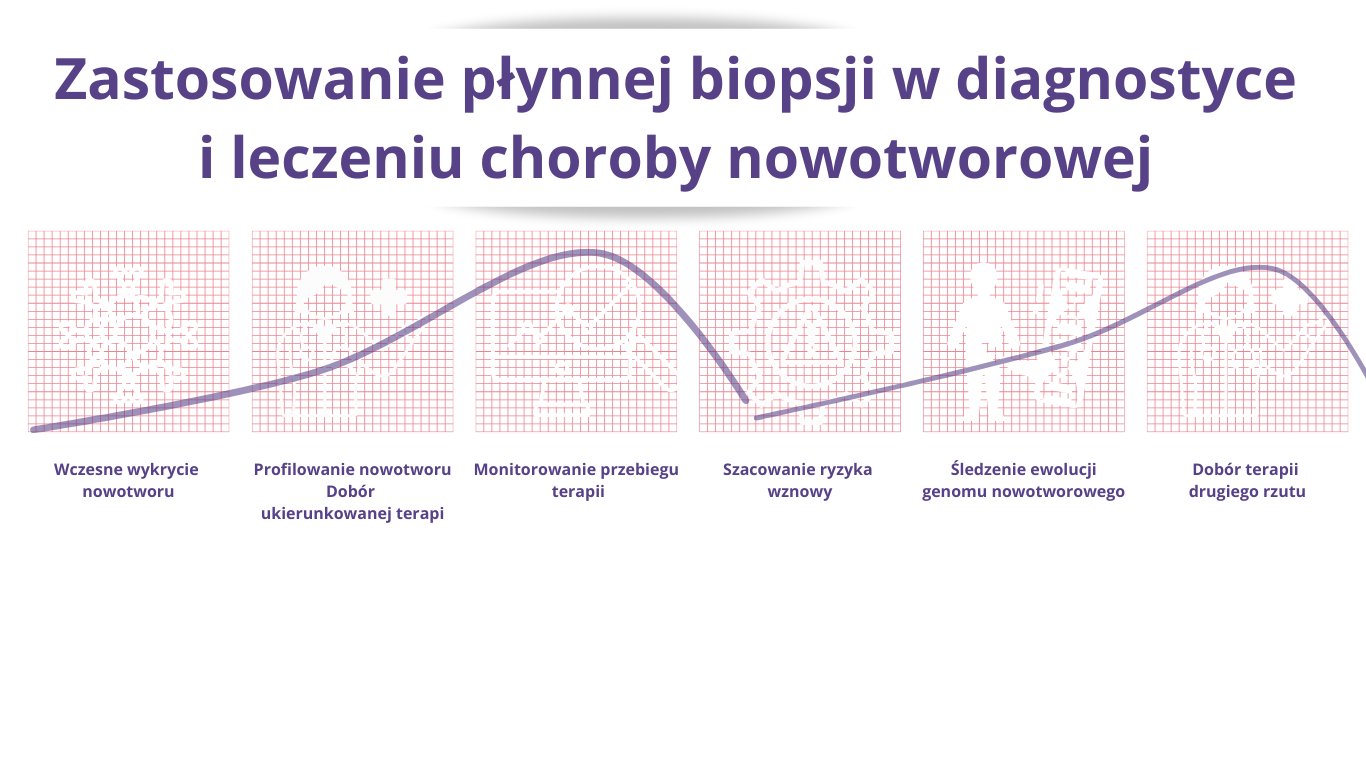
- Non-invasiveness – simplicity of sample collection (i.e. venous blood sample). the patient does not have to go through the stress and discomfort associated with traditional biopsy procedures. It can also be performed multiple times, allowing you to monitor disease progression in real time and adjust the treatment plan depending on the patient's response.
- Traditional biopsy is impossible in the case of numerous metastases to various organs,
- Possibility of detecting cancer at a very early stage (stage 1 of most cancers)
- The non-invasive nature of liquid biopsy allows it to be performed multiple times during treatment (therapy monitoring)
- Liquid biopsy is able to capture tumor heterogeneity to a much greater extent than traditional tissue biopsy. This means it can provide more information about the different genetic mutations that may occur in different parts of the same tumor.
- The material from a traditional (surgical) biopsy is most often damaged as a result of fixation with formalin and protection in paraffin. The material from the liquid biopsy is of good quality and allows for analysis using the NGS method.
Personalized Medicine
Personalized medicine, also known as precision medicine, is a paradigm that puts patient individuality at the forefront. It assumes that therapies should be adapted to the specific characteristics of the patient, including his or her genetic profile. In this context, liquid biopsy can be of great benefit.
The condition for using the most effective available treatment is to perform molecular profiling of the tumor. It can be performed with both traditional and liquid biopsy. There are databases collecting information on pathogenic gene variants associated with cancer processes, e.g. SANGER Institute COSMIC - Catalog of Somatic Mutations In Cancer. By understanding the genetic profile of the cancer, treatment based on known drugs can be optimally tailored to the patient's individual needs. The basic databases are:
- COSMIC – https://cancer.sanger.ac.uk/cosmic
- CIVIC – https://civicdb.org/home
- The Cancer Genome Atlas – https://cancergenome.nih.gov
Molecular diagnostic methods
Advanced molecular diagnostic methods have opened the door to personalized treatment in breast cancer, enabling the identification of genetic changes in various signaling pathways.
This enabled the creation of a molecular classification of breast cancer, with the following types: luminal A, luminal B, HER-positive, triple-negative TNBC, basal-like and TNBC with low claudin expression. Nevertheless, personalized treatment of breast cancer still relies on the assessment of well-known and thoroughly described immunohistochemical markers, such as estrogen and progesterone receptors and HER2 amplification.
For example, the presence of estrogen receptors is a criterion for qualifying patients for hormonal treatment with drugs such as tamoxifen, raloxifene, letrozole, anastrozole. In turn, approximately 20% breast cancer patients have HER2 amplification, suggesting a response to targeted drugs that block HER2 overexpression, such as trastuzumab (or herceptin), lapatinib, and pertuzumab.
In the treatment of colorectal cancer, monoclonal antibodies directed against the epidermal growth factor receptor (EGFR), such as panitumumab or cetuximab, can be used to treat metastases.
However, in the treatment of melanoma patients with the presence of the V600E activating mutation of the BRAF gene, the drugs of choice are BRAF inhibitors such as vemurafenib and dabrafenib.
When it comes to gastric stromal tumors (GIST), both in cases with a mutation activating the proto-oncogenes KIT or PD-GFRA in tumor cells, and in situations where these mutations do not occur, the use of a tyrosine kinase inhibitor - imitinib, is the therapeutic standard.
Lung cancer also benefits from personalized treatment methods. Thanks to the discovery of the presence of activating mutations in the genes encoding tyrosine kinases: EGFR and the ALK gene, it became possible to inhibit the "leading" molecular path in the development of non-small cell lung cancer. For patients with non-small cell lung cancer with a mutation activating the tyrosine kinase domain of the BRAF gene, gefitinib and erlotinib are used.
Below are examples of molecular profiling and an effective targeted drug:
Breast cancer – molecularly targeted drugs
| Gene | Variant | Name of the active substance (drug) | Additional information | Is it included in the European standard of treatment (according to ESMO)? |
| PIK3CA | H1047R E545K | Lapatinib | Lapatinib is an inhibitor of the intracellular tyrosine kinase domains of two cancer cell receptors: epidermal growth factor (EGFR, ErbB-1) and human epidermal growth factor HER2/neu (ErbB-2). | YES |
| ERBB2 | L755S R678Q D769H L755P T798M L638S V773L K753E | Lapatinib | Inhibition of the activity of these kinases by lapatinib leads to inhibition of cell division and apoptotic death of the cancer cell. | YES |
| PIK3CA | H1047R | Everolimus | Everolimus is a proliferation signal inhibitor. By inhibiting the activity of mTOR kinase, it limits the division of cancer cells and reduces the level of HIF and VEGF proteins in the tumor or its environment, inhibiting the development of tumor vascularization. | YES |
| STK11 | F354L | Everolimus | as above | YES |
| ESR1 | Y537S Y537N Y537C S463P E380Q L536R | Palbociclib | Palbociclib is a highly selective, reversible inhibitor of cyclin-dependent kinases (CDKs) 4 and 6, which regulate cell growth and division. This drug, administered together with aromatase inhibitors or fulvestrant, used in anticancer hormone therapy, reduces the ability of cancer cells to multiply. | YES |
| PIK3CA | H1047R E545K C420R K111N I391M | Kadcyla (trastuzumab emtansine) | This drug is composed of two interconnected active ingredients: a recombinant, humanized IgG1 monoclonal antibody that binds specifically to domain IV of the extracellular part of the HER2 receptor, and DM1 - a toxic substance that kills cells during their division and growth. The preparation is used to treat metastatic breast cancer with HER2 overexpression. In combination with other chemotherapy drugs, it significantly reduces the risk of disease recurrence and increases the degree of response to treatment. | YES |
| ERBB2 | V777L | Kadcyla (trastuzumab emtansine) | as above | YES |
| ERBB2 | L755S P780INS V842I V777L G309A R678Q R896C L755W D769H D769Y L869R T798I | Neratinib | Neratinib is a tyrosine kinase inhibitor that blocks enzymes responsible for cell growth. Neratinib attaches to HER proteins on cancer cells and blocks their action, stopping the cells from growing and preventing the cancer from coming back. | YES |
Colorectal cancer – molecularly targeted drugs
| Gene | Variant | Name of the active substance (drug) | Additional information | Is it included in the European standard of treatment (according to ESMO)? |
| BRAF | V600E | Regorafenib | Regorafenib is a protein kinase inhibitor. It blocks the action of VEGFR2-TIE2 tyrosine kinases, which play an important role in the development of the blood vessel system supplying cancer tumors and in the growth and development of cancer cells. By inhibiting the blood flow to the tumor, it prevents the growth and development of cancer cells | YES |
| KRAS | G13D G12V G12D G12C G12A G12R G12S | Regorafenib | as above | YES |
| PIK3CA | E542K | Regorafenib | as above | YES |
| FBXW7 | R505C R505H G579W R658Q | Regorafenib | as above | YES |
| KRAS | no mutations in codons 12, 51, 59, 117 and 146 | Cetuximab | Cetuximab is a chimeric IgG1 monoclonal antibody directed specifically against the epidermal growth factor receptor (EGFR). It blocks the binding of endogenous EGFR ligands, resulting in inhibition of receptor activity. EGFR signaling pathways are associated with the control of cell survival, cell cycle progression, angiogenesis, cell migration, and cell metastasis. | YES |
| NRAS | no mutations in codons 12, 13, 59, 61 and 117 | Cetuximab | as above | YES |
| BRAF | no V600E mutation | Cetuximab | as above | YES |
| BRAF | no V600E mutation | Panitumumab | Panitumumab is a recombinant human IgG2 monoclonal antibody that has high affinity and specificity for the human EGFR receptor, found on the surface of some cells, and blocks its action. As a result of this process, cancer cells stop receiving the signals transmitted by EGFR that are necessary for the cancer to multiply and spread to other parts of the body. Panitumumab inhibits the growth of cancer cells, induces apoptosis and reduces the production of interleukin 8 and vascular endothelial growth factor. | YES |
| KRAS | no mutations in codons 12, 13, 59, 51, 117 and 146 | Panitumumab | as above | YES |
| NRAS | no variant codons 12, 13, 59, 61 and 117 | Panitumumab | as above | YES |
Summary
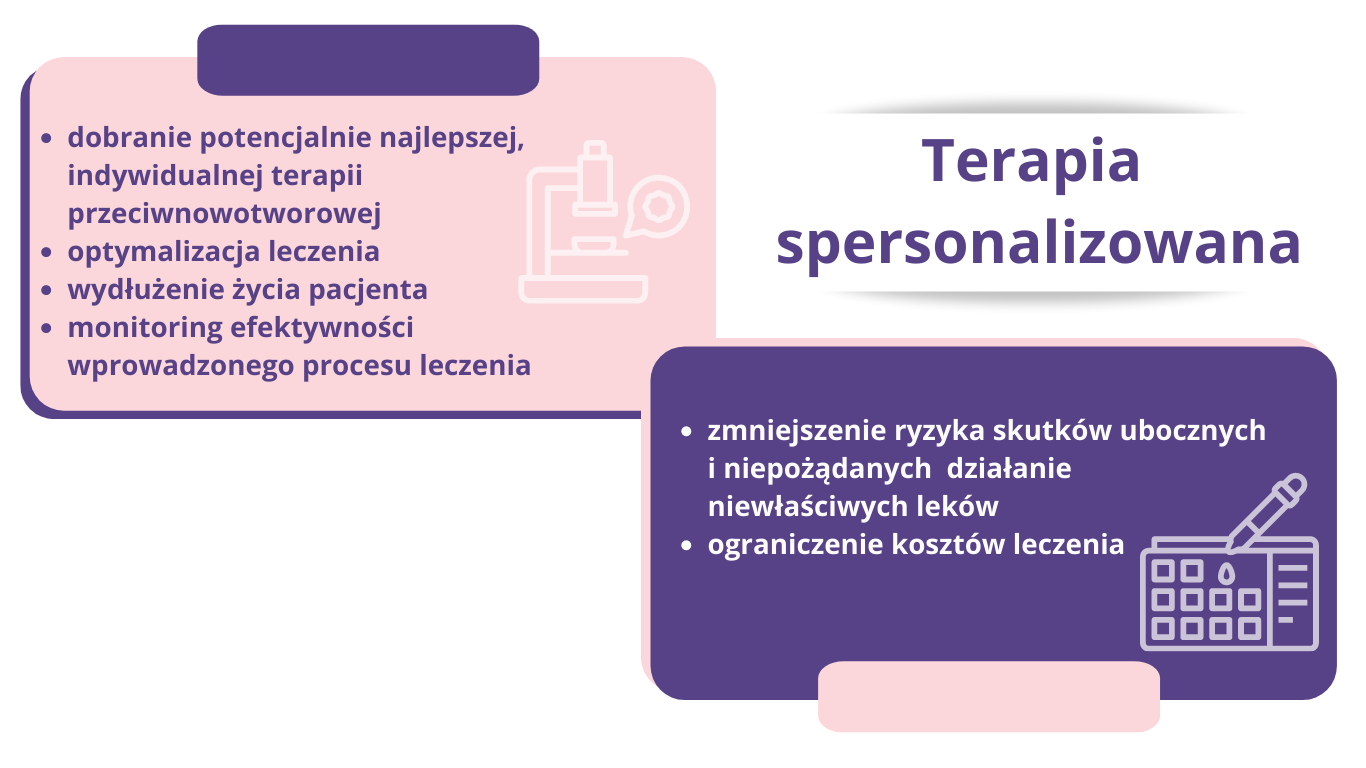
Personalized oncology brings new hope for cancer patients. Over recent years, scientists have accumulated a huge amount of knowledge about the functioning of cancer cells. They realized that cancer cells, for various reasons, had lost their natural metabolic balance, which led to their uncontrolled multiplication. Hence the concept of molecularly targeted therapy, which tries to restore balance in cancer cells. In recent years, new targeted drugs have appeared, depending on the genetic variant of the cancer. Molecular targeted therapy involves the use of drugs that target specific cellular mechanisms that are abnormal in cancer cells. These drugs are designed to act only on cancer cells, minimizing the effect on healthy cells. Many of them work by blocking specific receptors on the surface of cancer cells that are responsible for abnormal cellular functions.
Molecular targeted therapy is a promising strategy for cancer treatment. However, it is important to remember that each cancer is different and each patient is individual. Treatment must be tailored to the individual patient, and this requires thorough genetic evaluation and molecular profiling.
Liquid biopsy, thanks to its innovative sequencing techniques and the possibilities offered by personalized medicine, sets new standards in cancer diagnosis and therapy. This is a new and evolving approach, but it is full of promising possibilities. Liquid biopsy can provide many benefits, from reducing the risks associated with invasive procedures, to improving the effectiveness of therapy, to improving the quality of life of patients.
Literature:
The dawn of the liquid biopsy in the fight against cancer. Dominguez-Vigil IG et al., 2017, Oncotarget, Vol. 8, pp 291-2922
Clinical Validation of a Size-Based Microfluidic Device for Circulating Tumor Cell Isolation and Analysis in Renal Cell Carcinoma.
Leitão TP, Corredeira P, Kucharczak S, Rodrigues M, Piairo P, Rodrigues C, Alves P, Cavaco AM, Miranda M, Antunes M, Ferreira J, Palma Reis J, Lopes T, Diéguez L, Costa L. Int J Mol Sci . 2023 May 7;24(9):8404. doi: 10.3390/ijms24098404.
Clinical Evidence of Circulating Tumor DNA Application in Aggressive Breast Cancer.
El Hejjioui B, Bouguenouch L, Melhouf MA, El Mouhi H, Bennis S. Diagnostics (Basel). 2023 Jan 27;13(3):470. doi: 10.3390/diagnostics13030470.
The potential of liquid biopsy in the management of cancer patients. Markou A, Tzanikou E, Lianidou E. Semin Cancer Biol. 2022 Sep;84:69-79. doi: 10.1016/j.semcancer.2022.03.013. Epub 2022 Mar 21.
Diagnostic value of liquid biopsy in the era of precision medicine: 10 years of clinical evidence in cancer. Caputo V, Ciardiello F, Corte CMD, Martini G, Troiani T, Napolitano S. Explor Target Antitumor Ther. 2023;4(1):102-138. doi: 10.37349/etat.2023.00125. Epub 2023 Feb 28.
Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives. Peng Y, Mei W, Ma K, Zeng C. Front Oncol. 2021 Nov 18;11:763790. doi: 10.3389/fonc.2021.763790. eCollection 2021.
Heterogeneous mutation pattern in tumor tissue and circulating tumor DNA warrants parallel NGS panel testing. Guo Q, Wang J, Xiao J, Wang L, Hu X, Yu W, Song G, Lou J, Chen J. Mol Cancer. 2018 Aug 28;17(1):131. doi: 10.1186/s12943-018-0875-0
Using circulating cell-free DNA to monitor personalized cancer therapy. Oellerich M, Schütz E, Beck J, Kanzow P, Plowman PN, Weiss GJ, Walson PD. Crit Rev Clin Lab Sci. 2017 May;54(3):205-218. doi: 10.1080/10408363.2017.1299683. Epub 2017Apr 10.
A field guide for cancer diagnostics using cell-free DNA: From principles to practice and clinical applications. Volckmar AL, Sültmann H, Riediger A, Fioretos T, Schirmacher P, Endris V, Stenzinger A, Dietz S. Genes Chromosomes Cancer. 2018 Mar;57(3):123-139. doi: 10.1002/gcc.22517. Epub 2017 Dec 20.
The article was prepared together with the DNA Research Center: https://cbdna.pl/